Business Partner
Business Partner

Business Partner
To date, the rise of genomics had a significant impact on the understanding and practice of precision medicine. There have been several sequencing efforts throughout the years that have significantly increased genomics knowledge. Finding new variants and understand how these genetic variations are associated with cancer, researchers and pharmaceutical companies can use the information to develop new targeted therapies to select the best route of treatment for a patient.
A holistic knowledge of the needs of the healthcare system and cancer patients in Thailand, including the exploration of the Patient Journey and a deep understanding of the impact of advanced diagnostic technology, thus Gene agnosTic (GT) can be your partner in expansion the innovation for cancer patients in Thailand. We are hoping to utilize obvious synergies emerging from the need for a unified patient pathway incorporated in the clinical practice. Establishing successful partnership is essential for pharmaceutical companies looking to drive innovation, growth, and focus on the best outcome for patients.
Current findings of tumor marker tests and biomarkers provide more possible solutions for cancer patients. Below lists are the example of tumor marker in common use,
“Tumor Marker Tests in Common Use was originally published by the National Cancer Institute.” Source: The National Cancer Institute
Tumor Markers in Common Use
A tumor marker is anything present in or produced by cancer cells or other cells of the body in response to cancer or certain benign (noncancerous) conditions that provides information about a cancer, such as how aggressive it is, whether it can be treated with a targeted therapy, or whether it is responding to treatment. Listed below are tumor marker tests that are in common use, mainly to determine treatment or to help make a diagnosis of cancer. This list is not comprehensive; in particular, new tumor markers frequently become available and may not be included. This list also does not include the many tumor markers that are tested by immunophenotyping and immunohistochemistry to help diagnose cancer and to distinguish between different types of cancer. Some tumor markers listed below are targets for targeted therapy in multiple cancers but serve as tumor markers for only a subset of cancers. For each test, the main cancer types that it is used for are listed. Some markers may be used for other cancer types that are not listed.
ALK gene rearrangements and overexpression
Cancer types or cancer-like conditions: Non-small cell lung cancer, anaplastic large cell lymphoma, histiocytosis
What's analyzed: Tumor
How used: To help determine treatment and prognosis
B-cell immunoglobulin gene rearrangement
Cancer type: B-cell lymphoma
What's analyzed: Blood, bone marrow, or tumor tissue
How used: To help in diagnosis, to evaluate effectiveness of treatment, and to check for recurrence
BCL2 gene rearrangement
Cancer types: Lymphomas, leukemias
What’s analyzed: Blood, bone marrow, or tumor tissue
How used: For diagnosis and planning therapy
BCR-ABL fusion gene (Philadelphia chromosome)
Cancer types: Chronic myeloid leukemia, acute lymphoblastic leukemia, and acute myelogenous leukemia
What's analyzed: Blood or bone marrow
How used: To confirm diagnosis, predict response to targeted therapy, help determine treatment, and monitor disease status
BRAF V600 mutations
Cancer types or cancer-like conditions: Thyroid cancer, melanoma, ovarian cancer, Erdheim-Chester disease, Langerhans cell histiocytosis, hairy cell leukemia, colorectal cancer, non-small cell lung cancer, and some other cancer types
What's analyzed: Tumor
How used: To help determine treatment
BRCA1 and BRCA2 gene mutations
Cancer types: Breast, ovarian, pancreatic, and prostate cancers
What's analyzed: Blood and/or tumor
How used: To help determine treatment
C-kit/CD117
Cancer types: Gastrointestinal stromal tumor, mucosal melanoma, acute myeloid leukemia, and mast cell disease
What's analyzed: Tumor, blood, or bone marrow
How used: To help in diagnosis and to help determine treatment
MiCyclin D1 (CCND1) gene rearrangement or expression
Cancer types: Lymphoma, myeloma
What’s analyzed: Tumor
How used: To help in diagnosis
DPD gene mutation
Cancer types: Breast, colorectal, gastric, and pancreatic cancers
What's analyzed: Blood
How used: To predict the risk of a toxic reaction to 5-fluorouracil therapy
EGFR
Cancer types: Non-small cell lung cancer and colorectal cancer
What's analyzed: Tumor
How used: To help determine treatment and prognosis
ESR1 gene mutation
Cancer type: Breast cancer
What's analyzed: Tumor
How used: To help determine treatment
Estrogen receptor (ER)/progesterone receptor (PR)
Cancer type: Breast cancer
What's analyzed: Tumor
How used: To help determine treatment
FGFR2 and FGFR3 gene mutations
Cancer types: Bladder cancer and cholangiocarcinoma
What's analyzed: Tumor
How used: To help determine treatment
FLT3 gene mutations
Cancer type: Acute myeloid leukemia
What's analyzed: Blood
How used: To help determine treatment and prognosis
HER2/neu (ERBB2) gene amplification, mutations, protein overexpression
Cancer types: Breast, ovarian, bladder, pancreatic, non-small cell lung, gastroesophageal, and stomach cancers
What's analyzed: Tumor
How used: To help determine treatment
IDH1 and IDH2 gene mutations
Cancer types: Acute myeloid leukemia, cholangiocarcinoma, and glioma
What's analyzed: Bone marrow, tissue, or blood
How used: To help determine treatment
IRF4 gene rearrangement
Cancer type: Lymphoma
What’s analyzed: Tumor
How used: To help in diagnosis
JAK2 gene mutation
Cancer type: Certain types of leukemia
What's analyzed: Blood and bone marrow
How used: To help in diagnosis
KRAS gene mutation
Cancer types: Colorectal cancer and non-small cell lung cancer
What's analyzed: Tumor, plasma
How used: To help determine treatment
Microsatellite instability (MSI) and/or mismatch repair deficiency (dMMR)
Cancer types: Colorectal cancer, endometrial carcinoma, and other solid tumors
What's analyzed: Tumor
How used: To guide treatment and to identify those at high risk of having inherited certain cancer-predisposing syndromes
MYC gene expression
Cancer types: Lymphomas, leukemias
What’s analyzed: Tumor
How used: To help in diagnosis and to help determine treatment
MYD88 gene mutation
Cancer types: Lymphoma, Waldenström macroglobulinemia
What’s analyzed: Tumor
How used: To help in diagnosis and to help determine treatment
NTRK gene fusion
Cancer type: Any solid tumor
What’s analyzed: Tumor
How used: To help determine treatment
PIK3CA gene mutation status
Cancer types: Breast cancer, colorectal cancer, non-small cell lung cancer, and ovarian cancer
What's analyzed: Tumor
How used: To assess the likely course of the disease (prognosis) and to determine the appropriateness of treatment with specific targeted therapies
Programmed death ligand 1 (PD-L1)
Cancer types: Non-small cell lung cancer, triple-negative breast cancer, liver cancer, stomach cancer, gastroesophageal junction cancer, cervical cancer, bladder cancer, head and neck cancer, and classical Hodgkin lymphoma and other aggressive lymphoma subtypes
What's analyzed: Tumor
How used: To help determine treatment
RET gene fusions and mutations
Cancer type: Medullary thyroid cancer, thyroid cancer, and non-small cell lung cancer
What's analyzed: Tumor
How used: To help determine treatment
ROS1 gene rearrangement
Cancer type: Non-small cell lung cancer
What's analyzed: Tumor
How used: To help determine treatment
TP53 gene mutations
Cancer type: B-cell chronic lymphocytic leukemia
What's analyzed: Blood
How used: To help determine treatment and prognosis
Tumor mutational burden (TMB)
Cancer type: Solid tumors
What's analyzed: Tumor
How used: To help determine treatment
Reviewed: December 7, 2023
“List of Targeted Therapy Drugs Approved for Specific Types of Cancer was originally published by the National Cancer Institute.”
List of Targeted Therapy Drugs Approved for Specific Types of Cancer
The FDA has approved targeted therapy drugs for the treatment of some people with the following types of cancer. Some targeted therapy drugs are listed more than once because they have been approved to treat more than one type of cancer. The generic drug name is listed first, with a brand name in parentheses.
Targeted therapy approved for bladder cancer
atezolizumab (Tecentriq)
avelumab (Bavencio)
enfortumab vedotin-ejfv (Padcev)
erdafitinib (Balversa)
nivolumab (Opdivo)
nogapendekin alfa inbakicept-pmln (Anktiva)
pembrolizumab (Keytruda)
sacituzumab govitecan-hziy (Trodelvy)
Targeted therapy approved for brain cancer
belzutifan (Welireg)
bevacizumab (Avastin)
dabrafenib (Tafinlar)
everolimus (Afinitor)
tovorafenib (Ojemda)
trametinib (Mekinist)
Targeted therapy approved for breast cancer
abemaciclib (Verzenio)
ado-trastuzumab emtansine (Kadcyla)
alpelisib (Piqray)
anastrozole (Arimidex)
capivasertib (Truqap)
elacestrant dihydrochloride (Orserdu)
everolimus (Afinitor)
exemestane (Aromasin)
fam-trastuzumab deruxtecan-nxki (Enhertu)
fulvestrant (Faslodex)
lapatinib ditosylate (Tykerb)
letrozole (Femara)
margetuximab-cmkb (Margenza)
neratinib maleate (Nerlynx)
olaparib (Lynparza)
palbociclib (Ibrance)
pembrolizumab (Keytruda)
pertuzumab (Perjeta)
pertuzumab, trastuzumab, and hyaluronidase-zzxf (Phesgo)
ribociclib (Kisqali)
sacituzumab govitecan-hziy (Trodelvy)
talazoparib tosylate (Talzenna)
tamoxifen citrate (Soltamox)
toremifene (Fareston)
trastuzumab (Herceptin)
tucatinib (Tukysa)
Targeted therapy approved for cervical cancer
bevacizumab (Avastin)
pembrolizumab (Keytruda)
tisotumab vedotin-tftv (Tivdak)
Targeted therapy approved for colorectal cancer
bevacizumab (Avastin)
cetuximab (Erbitux)
encorafenib (Braftovi)
fruquintinib (Fruzalqa)
ipilimumab (Yervoy)
nivolumab (Opdivo)
panitumumab (Vectibix)
pembrolizumab (Keytruda)
ramucirumab (Cyramza)
regorafenib (Stivarga)
tucatinib (Tukysa)
ziv-aflibercept (Zaltrap)
Targeted therapy approved for dermatofibrosarcoma protuberans
imatinib mesylate (Gleevec)
Targeted therapy approved for endocrine and neuroendocrine tumors
avelumab (Bavencio)
iobenguane I 131 (Azedra)
lanreotide acetate (Somatuline Depot)
lutetium Lu 177-dotatate (Lutathera)
Targeted therapy approved for endometrial cancer
dostarlimab-gxly (Jemperli)
lenvatinib mesylate (Lenvima)
pembrolizumab (Keytruda)
Targeted therapy approved for esophageal cancer
fam-trastuzumab deruxtecan-nxki (Enhertu)
ipilimumab (Yervoy)
nivolumab (Opdivo)
pembrolizumab (Keytruda)
ramucirumab (Cyramza)
tislelizumab-jsgr (Tevimbra)
trastuzumab (Herceptin)
Targeted therapy approved for head and neck cancer
cetuximab (Erbitux)
nivolumab (Opdivo)
pembrolizumab (Keytruda)
toripalimab-tpzi (Loqtorzi)
Targeted therapy approved for gastrointestinal stromal tumor
avapritinib (Ayvakit)
imatinib mesylate (Gleevec)
regorafenib (Stivarga)
ripretinib (Qinlock)
sunitinib malate (Sutent)
Targeted therapy approved for giant cell tumor
denosumab (Xgeva)
pexidartinib hydrochloride (Turalio)
Targeted therapy approved for kidney cancer
avelumab (Bavencio)
axitinib (Inlyta)
belzutifan (Welireg)
bevacizumab (Avastin)
cabozantinib-s-malate (Cabometyx)
everolimus (Afinitor)
ipilimumab (Yervoy)
lenvatinib mesylate (Lenvima)
nivolumab (Opdivo)
pazopanib hydrochloride (Votrient)
pembrolizumab (Keytruda)
sorafenib tosylate (Nexavar)
sunitinib malate (Sutent)
temsirolimus (Torisel)
tivozanib hydrochloride (Fotivda)
Targeted therapy approved for leukemia
acalabrutinib (Calquence)
alemtuzumab (Campath)
asciminib hydrochloride (Scemblix)
avapritinib (Ayvakit)
blinatumomab (Blincyto)
bosutinib (Bosulif)
brexucabtagene autoleucel (Tecartus)
dasatinib (Sprycel)
duvelisib (Copiktra)
enasidenib mesylate (Idhifa)
gemtuzumab ozogamicin (Mylotarg)
gilteritinib fumarate (Xospata)
glasdegib maleate (Daurismo)
ibrutinib (Imbruvica)
idelalisib (Zydelig)
imatinib mesylate (Gleevec)
inotuzumab ozogamicin (Besponsa)
ivosidenib (Tibsovo)
lisocabtagene maraleucel (Breyanzi)
midostaurin (Rydapt)
moxetumomab pasudotox-tdfk (Lumoxiti)
nilotinib (Tasigna)
obinutuzumab (Gazyva)
ofatumumab (Arzerra)
olutasidenib (Rezlidhia)
pemigatinib (Pemazyre)
pirtobrutinib (Jaypirca)
ponatinib hydrochloride (Iclusig)
quizartinib dihydrochloride (Vanflyta)
rituximab (Rituxan)
rituximab and hyaluronidase human (Rituxan Hycela)
tagraxofusp-erzs (Elzonris)
tisagenlecleucel (Kymriah)
tretinoin (Vesanoid)
venetoclax (Venclexta)
zanubrutinib (Brukinsa)
Targeted therapy approved for liver and bile duct cancer
atezolizumab (Tecentriq)
bevacizumab (Avastin)
cabozantinib-s-malate (Cabometyx)
durvalumab (Imfinzi)
futibatinib (Lytgobi)
ipilimumab (Yervoy)
ivosidenib (Tibsovo)
lenvatinib mesylate (Lenvima)
nivolumab (Opdivo)
pembrolizumab (Keytruda)
pemigatinib (Pemazyre)
ramucirumab (Cyramza)
regorafenib (Stivarga)
sorafenib tosylate (Nexavar)
tremelimumab-actl (Imjudo)
Targeted therapy approved for lung cancer
adagrasib (Krazati)
afatinib dimaleate (Gilotrif)
alectinib (Alecensa)
amivantamab-vmjw (Rybrevant)
atezolizumab (Tecentriq)
bevacizumab (Avastin)
binimetinib (Mektovi)
brigatinib (Alunbrig)
capmatinib hydrochloride (Tabrecta)
cemiplimab-rwlc (Libtayo)
ceritinib (Zykadia)
crizotinib (Xalkori)
dabrafenib mesylate (Tafinlar)
dacomitinib (Vizimpro)
durvalumab (Imfinzi)
encorafenib (Braftovi)
entrectinib (Rozlytrek)
erlotinib hydrochloride (Tarceva)
fam-trastuzumab deruxtecan-nxki (Enhertu)
gefitinib (Iressa)
ipilimumab (Yervoy)
lorlatinib (Lorbrena)
necitumumab (Portrazza)
nivolumab (Opdivo)
osimertinib mesylate (Tagrisso)
pembrolizumab (Keytruda)
pralsetinib (Gavreto)
ramucirumab (Cyramza)
repotrectinib (Augtyro)
selpercatinib (Retevmo)
sotorasib (Lumakras)
tepotinib hydrochloride (Tepmetko)
trametinib dimethyl sulfoxide (Mekinist)
tremelimumab-actl (Imjudo)
Targeted therapy approved for lung cancer
adagrasib (Krazati)
afatinib dimaleate (Gilotrif)
alectinib (Alecensa)
amivantamab-vmjw (Rybrevant)
atezolizumab (Tecentriq)
bevacizumab (Avastin)
binimetinib (Mektovi)
brigatinib (Alunbrig)
capmatinib hydrochloride (Tabrecta)
cemiplimab-rwlc (Libtayo)
ceritinib (Zykadia)
crizotinib (Xalkori)
dabrafenib mesylate (Tafinlar)
dacomitinib (Vizimpro)
durvalumab (Imfinzi)
encorafenib (Braftovi)
entrectinib (Rozlytrek)
erlotinib hydrochloride (Tarceva)
fam-trastuzumab deruxtecan-nxki (Enhertu)
gefitinib (Iressa)
ipilimumab (Yervoy)
lorlatinib (Lorbrena)
necitumumab (Portrazza)
nivolumab (Opdivo)
osimertinib mesylate (Tagrisso)
pembrolizumab (Keytruda)
pralsetinib (Gavreto)
ramucirumab (Cyramza)
repotrectinib (Augtyro)
selpercatinib (Retevmo)
sotorasib (Lumakras)
tepotinib hydrochloride (Tepmetko)
trametinib dimethyl sulfoxide (Mekinist)
tremelimumab-actl (Imjudo)
Targeted therapy approved for lymphoma
acalabrutinib (Calquence)
axicabtagene ciloleucel (Yescarta)
belinostat (Beleodaq)
bexarotene (Targretin)
bortezomib (Velcade)
brentuximab vedotin (Adcetris)
brexucabtagene autoleucel (Tecartus)
crizotinib (Xalkori)
denileukin diftitox (Ontak)
duvelisib (Copiktra)
epcoritamab-bysp (Epkinly)
glofitamab-gxbm (Columvi)
ibritumomab tiuxetan (Zevalin)
ibrutinib (Imbruvica)
lisocabtagene maraleucel (Breyanzi)
loncastuximab tesirine-lpyl (Zynlonta)
mogamulizumab-kpkc (Poteligeo)
mosunetuzumab-axgb (Lunsumio)
nivolumab (Opdivo)
obinutuzumab (Gazyva)
pembrolizumab (Keytruda)
pemigatinib (Pemazyre)
pirtobrutinib (Jaypirca)
polatuzumab vedotin-piiq (Polivy)
pralatrexate (Folotyn)
rituximab (Rituxan)
rituximab and hyaluronidase human (Rituxan Hycela)
romidepsin (Istodax)
selinexor (Xpovio)
siltuximab (Sylvant)
tafasitamab-cxix (Monjuvi)
tazemetostat hydrobromide (Tazverik)
tisagenlecleucel (Kymriah)
venetoclax (Venclexta)
vorinostat (Zolinza)
zanubrutinib (Brukinsa)
Targeted therapy approved for malignant mesothelioma
ipilimumab (Yervoy)
nivolumab (Opdivo)
Targeted therapy approved for multiple myeloma
bortezomib (Velcade)
carfilzomib (Kyprolis)
ciltacabtagene autoleucel (Carvykti)
daratumumab (Darzalex)
daratumumab and hyaluronidase-fihj (Darzalex Faspro)
elranatamab-bcmm (Elrexfio)
elotuzumab (Empliciti)
idecabtagene vicleucel (Abecma)
isatuximab-irfc (Sarclisa)
ixazomib citrate (Ninlaro)
talquetamab-tgvs (Talvey)
selinexor (Xpovio)
teclistamab-cqyv (Tecvayli)
Targeted therapy approved for myelodysplastic and myeloproliferative disorders
fedratinib hydrochloride (Inrebic)
imatinib mesylate (Gleevec)
ivosidenib (Tibsovo)
momelotinib dihydrochloride monohydrate (Ojjaara)
pacritinib citrate (Vonjo)
pemigatinib (Pemazyre)
ruxolitinib phosphate (Jakafi)
Targeted therapy approved for neuroblastoma
dinutuximab (Unituxin)
naxitamab-gqgk (Danyelza)
Targeted therapy approved for ovarian epithelial, fallopian tube, and primary peritoneal cancers
bevacizumab (Avastin)
mirvetuximab soravtansine-gynx (Elahere)
niraparib tosylate monohydrate (Zejula)
olaparib (Lynparza)
rucaparib camsylate (Rubraca)
Targeted therapy approved for pancreatic cancer
belzutifan (Welireg)
erlotinib hydrochloride (Tarceva)
everolimus (Afinitor)
olaparib (Lynparza)
sunitinib malate (Sutent)
Targeted therapy approved for plexiform neurofibroma
selumetinib sulfate (Koselugo)
Targeted therapy approved for prostate cancer
abiraterone acetate (Zytiga)
apalutamide (Erleada)
cabazitaxel (Jevtana)
darolutamide (Nubeqa)
enzalutamide (Xtandi)
lutetium Lu 177 vipivotide tetraxetan (Pluvicto)
niraparib tosylate monohydrate and abiraterone acetate (Akeega)
olaparib (Lynparza)
talazoparib tosylate (Talzenna)
radium 223 dichloride (Xofigo)
rucaparib camsylate (Rubraca)
Targeted therapy approved for skin cancer
alitretinoin (Panretin)
atezolizumab (Tecentriq)
avelumab (Bavencio)
binimetinib (Mektovi)
cemiplimab-rwlc (Libtayo)
cobimetinib fumarate (Cotellic)
dabrafenib mesylate (Tafinlar)
encorafenib (Braftovi)
ipilimumab (Yervoy)
nivolumab (Opdivo)
nivolumab and relatlimab-rmbw (Opdualag)
pembrolizumab (Keytruda)
retifanlimab-dlwr (Zynyz)
sonidegib (Odomzo)
tebentafusp-tebn (Kimmtrak)
trametinib dimethyl sulfoxide (Mekinist)
vismodegib (Erivedge)
vemurafenib (Zelboraf)
Targeted therapy approved for soft tissue sarcoma
alitretinoin (Panretin)
atezolizumab (Tecentriq)
crizotinib (Xalkori)
nirogacestat hydrobromide (Ogsiveo)
pazopanib hydrochloride (Votrient)
sirolimus protein-bound particles (Fyarro)
tazemetostat hydrobromide (Tazverik
Targeted therapy approved for solid tumors anywhere in the body
dabrafenib mesylate (Tafinlar)
dostarlimab-gxly (Jemperli)
entrectinib (Rozlytrek)
fam-trastuzumab deruxtecan-nxki (Enhertu)
larotrectinib sulfate (Vitrakvi)
pembrolizumab (Keytruda)
selpercatinib (Retevmo)
trametinib dimethyl sulfoxide (Mekinist)
Targeted therapy approved for stomach (gastric) cancer
fam-trastuzumab deruxtecan-nxki (Enhertu)
nivolumab (Opdivo)
pembrolizumab (Keytruda)
ramucirumab (Cyramza)
trastuzumab (Herceptin)
Targeted therapy approved for systemic mastocytosis
avapritinib (Ayvakit)
imatinib mesylate (Gleevec)
midostaurin (Rydapt)
Targeted therapy approved for thyroid cancer
cabozantinib-s-malate (Cometriq)
dabrafenib mesylate (Tafinlar)
lenvatinib mesylate (Lenvima)
pralsetinib (Gavreto)
selpercatinib (Retevmo)
sorafenib tosylate (Nexavar)
trametinib dimethyl sulfoxide (Mekinist)
vandetanib (Caprelsa)
Updated: May 17, 2024
Leading innovators in cancer combination therapy for the pharmaceutical industry
The pharmaceutical industry continues to be a hotbed of patent innovation. Activity is driven by the evolution of treatment paradigms, and the gravity of unmet needs, as well as the growing importance of technologies such as pharmacogenomics, digital therapeutics, and artificial intelligence. In the last three years alone, there have been over 787,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in pharma: cancer combination therapy.
Cancer combination therapy is a key innovation area in the pharmaceutical industry
Cancer combination therapy refers to the use of multiple drugs or treatment modalities in combination to increase the efficacy of cancer treatment and improve patient outcomes. This may involve the use of different drug classes or therapies that target different aspects of cancer cells, such as angiogenesis inhibitors, chemotherapy agents, and immunotherapies.
GlobalData’s analysis also uncovers the companies at the forefront of each innovation area and assesses the potential reach and impact of their patenting activity across different applications and geographies. According to GlobalData, there are 1020+ companies, spanning technology vendors, established pharmaceutical companies, and up-and-coming start-ups engaged in the development and application of cancer combination therapy.
Key players in cancer combination therapy – a disruptive innovation in the pharmaceutical industry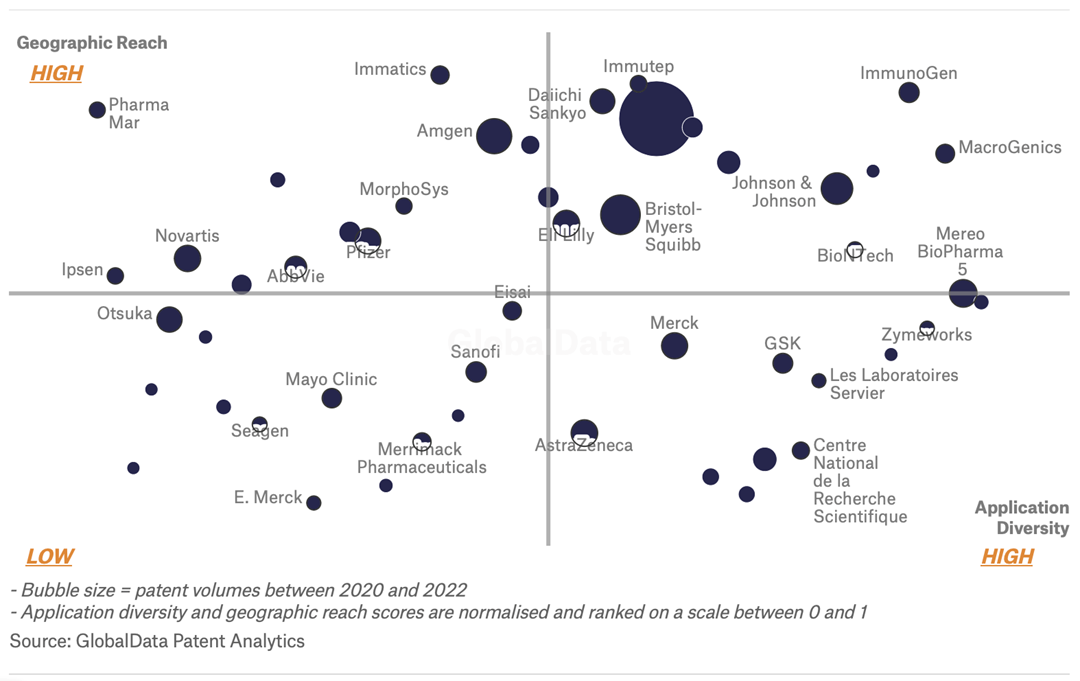
‘Application diversity’ measures the number of applications identified for each patent. It broadly splits companies into either ‘niche’ or ‘diversified’ innovators.
‘Geographic reach’ refers to the number of countries each patent is registered in. It reflects the breadth of geographic application intended, ranging from ‘global’ to ‘local’.
12 September 2024
Viewed 1562 times











